Finding variants in haploid genomes
Introduction
The majority of life on Earth is non-diploid and represented by prokaryotes, viruses and their derivatives such as our own mitochondria or plant's chloroplasts. In non-diploid systems allele frequencies can range anywhere between 0 and 100% and there could be multiple (not just two) alleles per locus. The main challenge associated with non-diploid variant calling is the difficulty in distinguishing between sequencing noise (abundant in all NGS platforms) and true low frequency variants. Some of the early attempts to do this well have been accomplished on human mitochondrial DNA although the same approaches will work equally good on viral and bacterial genomes:
- 2014 - Maternal age effect and severe germ-line bottleneck in the inheritance of human mitochondrial DNA
- 2015 - Extensive tissue-related and allele-related mtDNA heteroplasmy suggests positive selection for somatic mutations.
As an example of non-diploid system we will be using human mitochondrial genome as an example. However, this approach will also work for most bacterial and viral genomes as well.
There are two ways one can call variants:
- By comparing reads against an existing genome assembly
- By assembling genome first and then mapping against that assembly
 |
| This figure from a manuscript by Olson:2015 contrasts the two approaches. |
In this tutorials we will take the first path is which we map reads against an existing assembly. Later in the course (after we learn about assembly approaches) we will try the second approach as well.
The goal of this example is to detect heteroplasmies (variants within mitochondrial DNA). Mitochondria is transmitted maternally and heteroplasmy frequencies may change dramatically and unpredictably during the transmission, due to a germ-line bottleneck Cree:2008. As we mentioned above the procedure for finding variants in bacterial or viral genomes will be essentially the same.
A Galaxy Library contains datasets representing a child and a mother. These datasets are obtained by paired-end Illumina sequencing of human genomic DNA enriched for mitochondria. The enrichment was performed using long-range PCR with two primer pairs that amplify the entire mitochondrial genome. This means that these samples still contain a lot of DNA from the nuclear genome, which, in this case, is a contaminant.
Importing example datasets
For this tutorial we have prepared a subset of data previously published by our group. Let's import these data into Galaxy.
Data upload from a Galaxy Library
- Go to this this Galaxy library
- You will see screen like the one shown above
- Click to History button.
- Galaxy will prompt you to ask whether you want to import these data into already existing or new history.
- It is better to create a new history, so type some descriptive name within
or create newtext field- click Import
- A green message will appear once the import is done. Click on it and will see the history you have just created. It will be populated with the four datasets as shown below:
QC'ing the data
Before proceeding with the analysis, we need to find out how good the data actually is. For this will use FastQC tool that can be found in NGS: QC and manipulation → FastQC section of Galaxy tools:
Quality Control of the data
QC'ing reads using FastQC. Note that we selected all four datasets at once by pressing the middle button
adjacent to the Short read data from your current history widget. Once
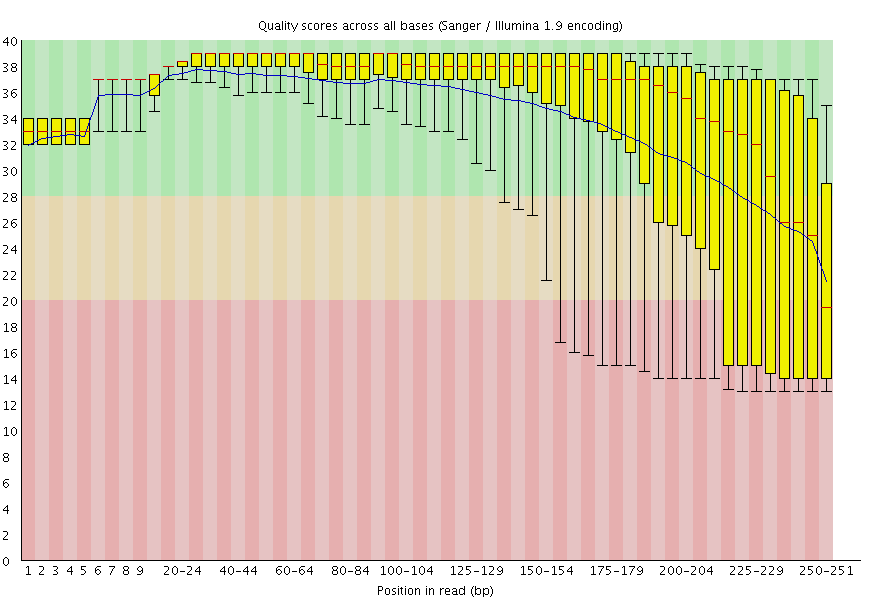
FastQCjob runs, you will be able to look at the HTML reports generated by this tool.The data have generally high quality in this example:
FastQC plot for one of the mitochondrial datasets shows that qualities are acceptable for 250 bp reads (mostly in the green, which is at or above phred score of 30).
Mapping the reads
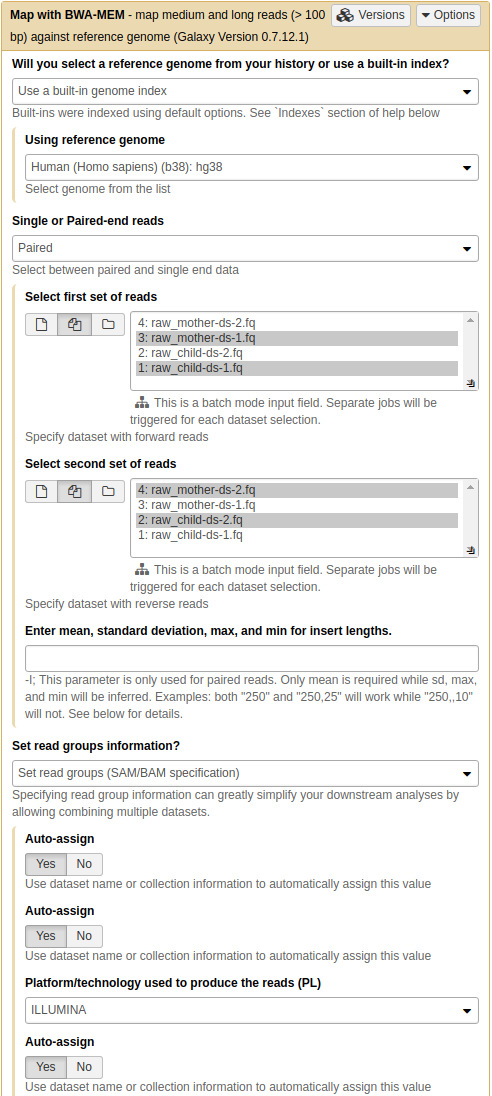
Our reads are long (250 bp) and as a result we will be using bwa mem to align them against the reference genome as it has good mapping performance for longer reads (100bp and up).
Mapping with
bwa mem
Running
bwa memon our datasets. Look carefully at parameter settings:
- We select
hg38version of the human genome as the reference- By using the middle button again
we select datasets 1 and 3 as Select the first set of reads and datasets 2 and 4 as Select the second set of reads. Galaxy will automatically launch two bwa-mem jobs using datasets 1,2 and 3,4 generating two resulting BAM files.
- By setting Set read groups information to
Set read groups (SAM/BAM specifications)and clicking Auto-assign we will ensure that the reads in the resulting BAM dataset are properly set.
Merging BAM datasets
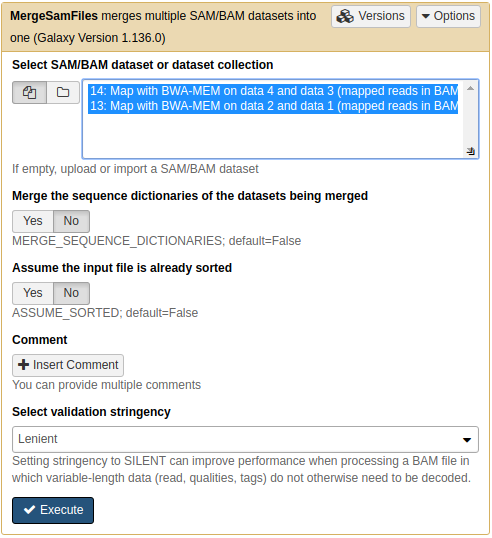
Because we have set read groups, we can now merge the two BAM dataset into one. This is because read groups label each read as belonging to either mother or child.
We can BAM dataset using NGS: Picard → MergeSAMFiles tool:
Merging multiple datasets into one
Merging two BAM datasets into one. Note that two inputs are highlighted.
Removing duplicates
Preparation of sequencing libraries (at least at the time of writing) for technologies such as Illumina (used in this example) involves PCR amplification. It is required to generate sufficient number of sequencing templates so that a reliable detection can be performed by base callers. Yet PCR has it's biases, which are especially profound in cases of multitemplate PCR used for construction of sequencing libraries (Kanagawa et al. 2003).
Duplicates can be identified based on their outer alignment coordinates or using sequence-based clustering. One of the common ways for identification of duplicate reads is the MarkDuplicates utility from Picard package. It is designed to identify both PCR and optical duplicates (the following is an excerpt from Picard documentation):
Duplicates are identified as read pairs having identical 5' positions (coordinate and strand) for both reads in a mate pair (and optionally, matching unique molecular identifier reads; see BARCODE_TAG option). Optical, or more broadly Sequencing, duplicates are duplicates that appear clustered together spatially during sequencing and can arise from optical/imagine-processing artifacts or from bio-chemical processes during clonal amplification and sequencing; they are identified using the READ_NAME_REGEX and the OPTICAL_DUPLICATE_PIXEL_DISTANCE options. The tool's main output is a new SAM or BAM file in which duplicates have been identified in the SAM flags field, or optionally removed (see REMOVE_DUPLICATE and REMOVE_SEQUENCING_DUPLICATES), and optionally marked with a duplicate type in the 'DT' optional attribute. In addition, it also outputs a metrics file containing the numbers of READ_PAIRS_EXAMINED, UNMAPPED_READS, UNPAIRED_READS, UNPAIRED_READ DUPLICATES, READ_PAIR_DUPLICATES, and READ_PAIR_OPTICAL_DUPLICATES. Usage example: java -jar picard.jar MarkDuplicates I=input.bam \ O=marked_duplicates.bam M=marked_dup_metrics.txt.
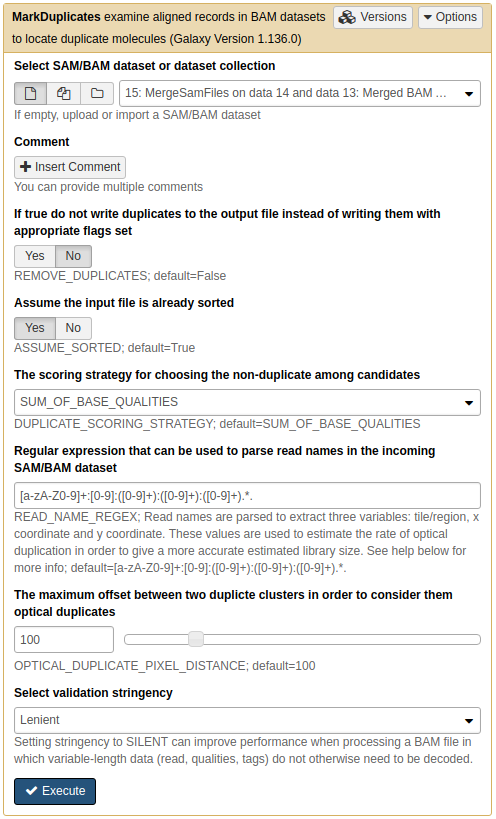
Let's use NGS: Picard → MarkDuplicates tool:
De-duplicating mapped data
De-duplicating the merged BAM dataset
MarkDuplicates produces a BAM dataset with duplicates removed and also a metrics file. Let's take a look at the metrics data:
raw_child-ds- 55 27551 55 50 1658 0 0.061026 219628
raw_mother-ds- 96 54972 96 90 4712 0 0.086459 302063
where columns are:
- LIBRARY (read group in our case)
- UNPAIRED_READS_EXAMINED
- READ_PAIRS_EXAMINED-
- UNMAPPED_READS
- UNPAIRED_READ_DUPLICATES
- READ_PAIR_DUPLICATES
- READ_PAIR_OPTICAL_DUPLICATES
- PERCENT_DUPLICATION
- ESTIMATED_LIBRARY_SIZE
In other words the two datasets had ~6% and ~9% duplicates, respectively.
Left-aligning indels
Left aligning of indels (a variant of re-aligning) is extremely important for obtaining accurate variant calls. This concept, while not difficult, requires some explanation. For illustrating how left-aligning works we expanded on an example provided by Tan:2015. Suppose you have a reference sequence and a sequencing read:
Reference GGGCACACACAGGG
Read GGGCACACAGGG
If you look carefully you will see that the read is simply missing a CA repeat. But it is not apparent to a mapper, so some of possible alignments and corresponding variant calls include:
Alignment Variant Call
GGGCACACACAGGG Ref: CAC
GGGCAC--ACAGGG Alt: C
GGGCACACACAGGG Ref: ACA
GGGCA--CACAGGG Alt: A
GGGCACACACAGGG Ref: GCA
GGG--CACACAGGG Alt: G
The last of these is left-aligned. In this case gaps (dashes) as moved as far left as possible (for a formal definition of left-alignment and variant normalization see Tan:2015).
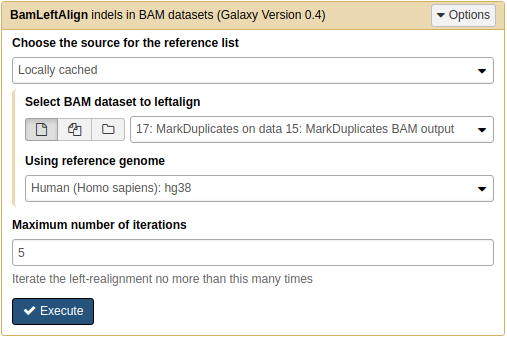
Let's perform left alignment using NGS: Variant Analysis → BamLeftAlign:
Left-aligning indels
Left-aligning a de-duplicated BAM dataset
Filtering reads
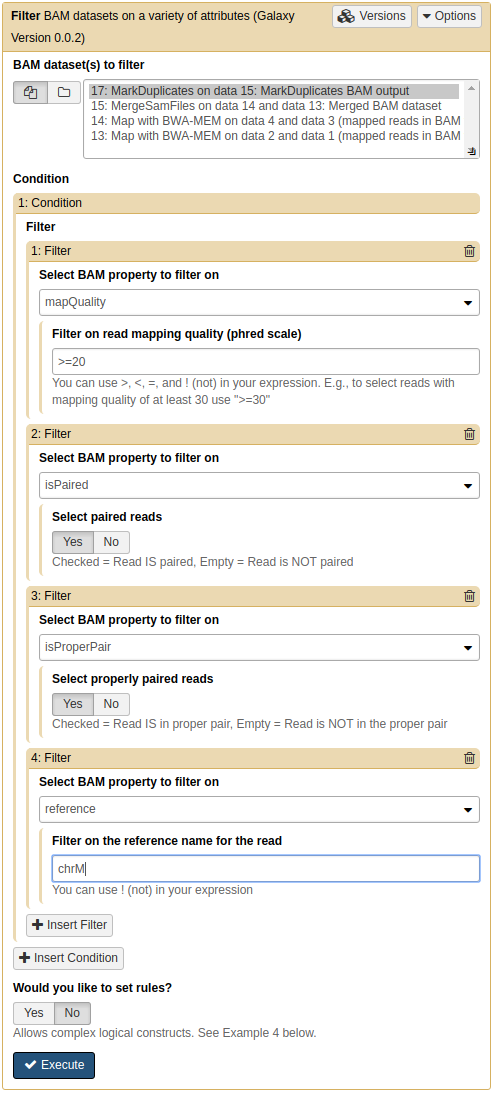
Remember that we are trying to call variants in mitochondrial genome. Let focus only on the reads derived from mitochondria genome by filtering everything else out. For this we will use NGS: BamTools → Filter:
Filtering BAM data
Filtering reads. There are several important point to note here:
- mapQuality is set to ⋝ 20 Mapping quality reflects the probability that the read is placed incorrectly. It uses phred scale. Thus 20 is 1/100 or 1% chance that the read is incorrectly mapped. By setting this parameter to ⋝ 20 we will keep all reads that have 1% or less probability of being mapped incorrectly.
- isPaired will eliminate singleton (unpaired) reads (make sure Yes is clicked on)
- isProperPair will only keep reads that map to the same chromosome and are properly placed (again, make sure Yes is clicked)
- reference is set to chrM
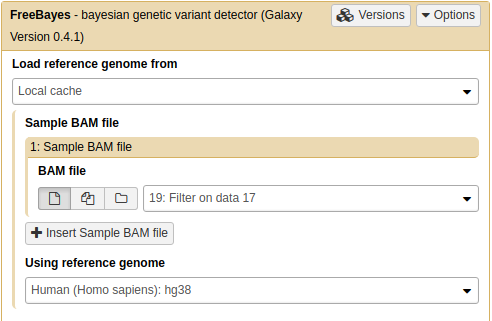
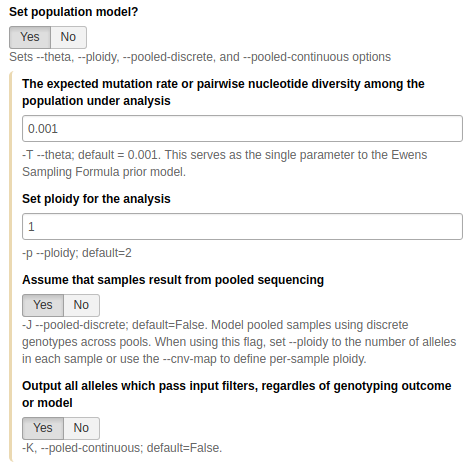
Calling non-diploid variants with FreeBayes
FreeBayes is widely used for calling variants in diploid systems. However, it can also be used for calling variants in pooled samples where the number of samples is not known. This is the exact scenario we have here: in our sample we have multiple mitochondrial (or bacterial or viral) genomes but we do not know exactly how many. Thus we will use the --pooled-continuous option of FreeBayes to generate frequency-based variant calls as well as some other options highlighted below (the tool is in NGS: Variant Analysis → FreeBayes):
Running
FreeBayes
Set genome to
hg38(the latest version)
Set regions to
chrMfrom1to16000. This will simply save us time since we are only interested in mitochondrial variants anyway
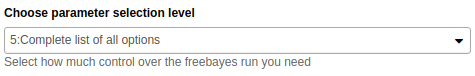
Choose
Complete list of all samplesfrom Choose parameter selection level drop down.
This is one of the most important parameter choices one needs to make when calling variants in non-diploid systems. Here set Set population model to
Yesand then:
- Set ploidy to
1- Set Assume that samples result from pooled sequencing to
Yes- Set Output all alleles which pass input filters, regardless of genotyping outcome or model to
Yes
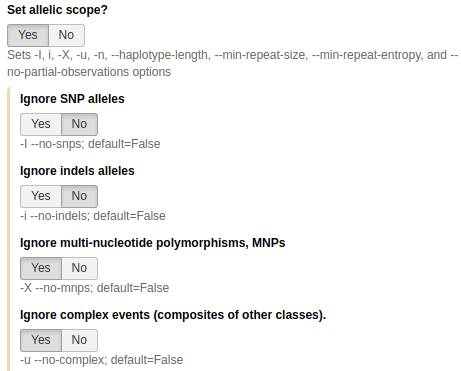
We will also set Allelic scope to
Yesand restrict variant types to single nucleotide polymorphisms only by:
- Keeping Ignore SNP alleles and Ignore indels alleles set to
No- Setting Ignore MNPs and Ignore complex events to
YesMitochondria has a number of low complexity regions (mononucleotide repeats). Setting these parameters as described above will decrease noise from these regions.
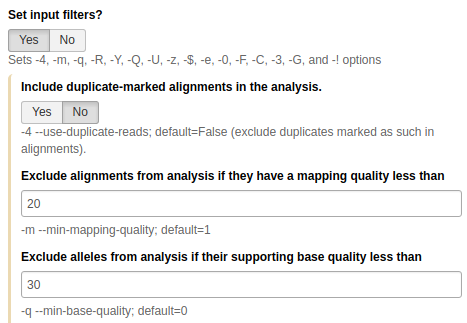
Finally, let's set Input filters to
Yesand set:
- Exclude alignments from analysis if they have a mapping quality less than to
20(phred score of 20). This will make FreeBayes to only consider reliably aligned reads.- Exclude alleles from analysis if their supporting base quality less than to
30(phred score of 30). This will make FreeBayes to only consider high quality bases.
This will produce a VCF dataset shows below (you may need to scroll sideways to see it in full). It lists 30 sites of interest (everything starting with a # is a comment):
Chrom Pos ID Ref Alt Qual Filter Info Format data
##fileformat=VCFv4.1
##fileDate=20161108
##source=freeBayes v0.9.20
##reference=/galaxy/data/hg38/sam_index/hg38.fa
##phasing=none
##commandline="freebayes --bam localbam_0.bam --fasta-reference /galaxy/data/hg38/sam_index/hg38.fa --vcf /galaxy-repl/main/files/017/782/dataset_17782376.dat --region chrM:1..16000"
##INFO=<ID=NS,Number=1,Type=Integer,Description="Number of samples with data">
##INFO=<ID=DP,Number=1,Type=Integer,Description="Total read depth at the locus">
##INFO=<ID=DPB,Number=1,Type=Float,Description="Total read depth per bp at the locus; bases in reads overlapping / bases in haplotype">
##INFO=<ID=AC,Number=A,Type=Integer,Description="Total number of alternate alleles in called genotypes">
##INFO=<ID=AN,Number=1,Type=Integer,Description="Total number of alleles in called genotypes">
##INFO=<ID=AF,Number=A,Type=Float,Description="Estimated allele frequency in the range (0,1]">
##INFO=<ID=RO,Number=1,Type=Integer,Description="Reference allele observation count, with partial observations recorded fractionally">
##INFO=<ID=AO,Number=A,Type=Integer,Description="Alternate allele observations, with partial observations recorded fractionally">
##INFO=<ID=PRO,Number=1,Type=Float,Description="Reference allele observation count, with partial observations recorded fractionally">
##INFO=<ID=PAO,Number=A,Type=Float,Description="Alternate allele observations, with partial observations recorded fractionally">
##INFO=<ID=QR,Number=1,Type=Integer,Description="Reference allele quality sum in phred">
##INFO=<ID=QA,Number=A,Type=Integer,Description="Alternate allele quality sum in phred">
##INFO=<ID=PQR,Number=1,Type=Float,Description="Reference allele quality sum in phred for partial observations">
##INFO=<ID=PQA,Number=A,Type=Float,Description="Alternate allele quality sum in phred for partial observations">
##INFO=<ID=SRF,Number=1,Type=Integer,Description="Number of reference observations on the forward strand">
##INFO=<ID=SRR,Number=1,Type=Integer,Description="Number of reference observations on the reverse strand">
##INFO=<ID=SAF,Number=A,Type=Integer,Description="Number of alternate observations on the forward strand">
##INFO=<ID=SAR,Number=A,Type=Integer,Description="Number of alternate observations on the reverse strand">
##INFO=<ID=SRP,Number=1,Type=Float,Description="Strand balance probability for the reference allele: Phred-scaled upper-bounds estimate of the probability of observing the deviation between SRF and SRR given E(SRF/SRR) ~ 0.5, derived using Hoeffding's inequality">
##INFO=<ID=SAP,Number=A,Type=Float,Description="Strand balance probability for the alternate allele: Phred-scaled upper-bounds estimate of the probability of observing the deviation between SAF and SAR given E(SAF/SAR) ~ 0.5, derived using Hoeffding's inequality">
##INFO=<ID=AB,Number=A,Type=Float,Description="Allele balance at heterozygous sites: a number between 0 and 1 representing the ratio of reads showing the reference allele to all reads, considering only reads from individuals called as heterozygous">
##INFO=<ID=ABP,Number=A,Type=Float,Description="Allele balance probability at heterozygous sites: Phred-scaled upper-bounds estimate of the probability of observing the deviation between ABR and ABA given E(ABR/ABA) ~ 0.5, derived using Hoeffding's inequality">
##INFO=<ID=RUN,Number=A,Type=Integer,Description="Run length: the number of consecutive repeats of the alternate allele in the reference genome">
##INFO=<ID=RPP,Number=A,Type=Float,Description="Read Placement Probability: Phred-scaled upper-bounds estimate of the probability of observing the deviation between RPL and RPR given E(RPL/RPR) ~ 0.5, derived using Hoeffding's inequality">
##INFO=<ID=RPPR,Number=1,Type=Float,Description="Read Placement Probability for reference observations: Phred-scaled upper-bounds estimate of the probability of observing the deviation between RPL and RPR given E(RPL/RPR) ~ 0.5, derived using Hoeffding's inequality">
##INFO=<ID=RPL,Number=A,Type=Float,Description="Reads Placed Left: number of reads supporting the alternate balanced to the left (5') of the alternate allele">
##INFO=<ID=RPR,Number=A,Type=Float,Description="Reads Placed Right: number of reads supporting the alternate balanced to the right (3') of the alternate allele">
##INFO=<ID=EPP,Number=A,Type=Float,Description="End Placement Probability: Phred-scaled upper-bounds estimate of the probability of observing the deviation between EL and ER given E(EL/ER) ~ 0.5, derived using Hoeffding's inequality">
##INFO=<ID=EPPR,Number=1,Type=Float,Description="End Placement Probability for reference observations: Phred-scaled upper-bounds estimate of the probability of observing the deviation between EL and ER given E(EL/ER) ~ 0.5, derived using Hoeffding's inequality">
##INFO=<ID=DPRA,Number=A,Type=Float,Description="Alternate allele depth ratio. Ratio between depth in samples with each called alternate allele and those without.">
##INFO=<ID=ODDS,Number=1,Type=Float,Description="The log odds ratio of the best genotype combination to the second-best.">
##INFO=<ID=GTI,Number=1,Type=Integer,Description="Number of genotyping iterations required to reach convergence or bailout.">
##INFO=<ID=TYPE,Number=A,Type=String,Description="The type of allele, either snp, mnp, ins, del, or complex.">
##INFO=<ID=CIGAR,Number=A,Type=String,Description="The extended CIGAR representation of each alternate allele, with the exception that '=' is replaced by 'M' to ease VCF parsing. Note that INDEL alleles do not have the first matched base (which is provided by default, per the spec) referred to by the CIGAR.">
##INFO=<ID=NUMALT,Number=1,Type=Integer,Description="Number of unique non-reference alleles in called genotypes at this position.">
##INFO=<ID=MEANALT,Number=A,Type=Float,Description="Mean number of unique non-reference allele observations per sample with the corresponding alternate alleles.">
##INFO=<ID=LEN,Number=A,Type=Integer,Description="allele length">
##INFO=<ID=MQM,Number=A,Type=Float,Description="Mean mapping quality of observed alternate alleles">
##INFO=<ID=MQMR,Number=1,Type=Float,Description="Mean mapping quality of observed reference alleles">
##INFO=<ID=PAIRED,Number=A,Type=Float,Description="Proportion of observed alternate alleles which are supported by properly paired read fragments">
##INFO=<ID=PAIREDR,Number=1,Type=Float,Description="Proportion of observed reference alleles which are supported by properly paired read fragments">
##INFO=<ID=technology.ILLUMINA,Number=A,Type=Float,Description="Fraction of observations supporting the alternate observed in reads from ILLUMINA">
##FORMAT=<ID=GT,Number=1,Type=String,Description="Genotype">
##FORMAT=<ID=GQ,Number=1,Type=Float,Description="Genotype Quality, the Phred-scaled marginal (or unconditional) probability of the called genotype">
##FORMAT=<ID=GL,Number=G,Type=Float,Description="Genotype Likelihood, log10-scaled likelihoods of the data given the called genotype for each possible genotype generated from the reference and alternate alleles given the sample ploidy">
##FORMAT=<ID=DP,Number=1,Type=Integer,Description="Read Depth">
##FORMAT=<ID=RO,Number=1,Type=Integer,Description="Reference allele observation count">
##FORMAT=<ID=QR,Number=1,Type=Integer,Description="Sum of quality of the reference observations">
##FORMAT=<ID=AO,Number=A,Type=Integer,Description="Alternate allele observation count">
##FORMAT=<ID=QA,Number=A,Type=Integer,Description="Sum of quality of the alternate observations">
#CHROM POS ID REF ALT QUAL FILTER INFO FORMAT raw_child-ds- raw_mother-ds-
chrM 73 . A G 33368.5 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=1095;CIGAR=1X;DP=1098;DPB=1098;DPRA=0;EPP=107.005;EPPR=5.18177;GTI=0;LEN=1;MEANALT=1.5;MQM=55.7744;MQMR=60;NS=2;NUMALT=1;ODDS=509.945;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=37602;QR=37;RO=1;RPL=359;RPP=284.863;RPPR=5.18177;RPR=736;RUN=1;SAF=507;SAP=16.0213;SAR=588;SRF=0;SRP=5.18177;SRR=1;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:273:0:0:273:9187:-827.167,-82.1812,0 1/1:825:1:37:822:28415:-2554.14,-241.429,0
chrM 263 . A G 13774.9 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=508;CIGAR=1X;DP=524;DPB=524;DPRA=0;EPP=39.1901;EPPR=0;GTI=0;LEN=1;MEANALT=2.5;MQM=60;MQMR=0;NS=2;NUMALT=1;ODDS=255.818;PAIRED=1;PAIREDR=0;PAO=0;PQA=0;PQR=0;PRO=0;QA=15693;QR=0;RO=0;RPL=373;RPP=245.138;RPPR=0;RPR=135;RUN=1;SAF=219;SAP=23.9556;SAR=289;SRF=0;SRP=0;SRR=0;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:154:0:0:150:4661:-419.661,-45.1545,0 1/1:370:0:0:358:11032:-993.047,-107.769,0
chrM 309 . CT CCTC,CC 4399.1 . AB=0.56535,0.285714;ABP=15.2141,134.229;AC=2,2;AF=0.5,0.5;AN=4;AO=186,94;CIGAR=1M2I1X,1M1X;DP=329;DPB=555.5;DPRA=0,0;EPP=23.6043,97.6311;EPPR=31.9633;GTI=0;LEN=3,1;MEANALT=6,6;MQM=60,59.8085;MQMR=60;NS=2;NUMALT=2;ODDS=89.3381;PAIRED=1,1;PAIREDR=1;PAO=13.3333,13.3333;PQA=339,339;PQR=290;PRO=11.3333;QA=4084,2577;QR=686;RO=30;RPL=114,78;RPP=23.6043,91.8097;RPPR=38.0434;RPR=72,16;RUN=1,1;SAF=0,63;SAP=406.904,26.6655;SAR=186,31;SRF=21;SRP=13.4334;SRR=9;TYPE=complex,snp;technology.ILLUMINA=1,1 GT:DP:RO:QR:AO:QA:GL 1/2:93:6:123:59,23:1308,638:-159.543,-53.5005,-52.9104,-105.161,0,-113.176 1/2:236:24:563:127,71:2776,1939:-368.987,-136.961,-169.835,-200.763,0,-245.13
chrM 513 . GCACACACACAC GCACACACACACAC 3522.72 . AB=0.647399;ABP=35.6577;AC=3;AF=0.75;AN=4;AO=156;CIGAR=1M2I11M;DP=231;DPB=321.083;DPRA=0;EPP=75.17;EPPR=5.48477;GTI=1;LEN=2;MEANALT=13.5;MQM=60;MQMR=60;NS=2;NUMALT=1;ODDS=3.87694;PAIRED=1;PAIREDR=1;PAO=46.5;PQA=1383.5;PQR=1383.5;PRO=46.5;QA=4585;QR=1403;RO=43;RPL=39;RPP=87.6977;RPPR=3.0608;RPR=117;RUN=1;SAF=111;SAP=63.6445;SAR=45;SRF=26;SRP=7.10075;SRR=17;TYPE=ins;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:58:7:225:44:1251:-105.403,0,-13.0389 0/1:173:36:1178:112:3334:-290.141,0,-96.0932
chrM 750 . A G 51447.4 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=1722;CIGAR=1X;DP=1736;DPB=1736;DPRA=0;EPP=3.03048;EPPR=20.3821;GTI=0;LEN=1;MEANALT=3;MQM=59.8868;MQMR=60;NS=2;NUMALT=1;ODDS=753.623;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=57871;QR=122;RO=8;RPL=720;RPP=103.291;RPPR=12.7819;RPR=1002;RUN=1;SAF=1151;SAP=427.217;SAR=571;SRF=1;SRP=12.7819;SRR=7;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:436:4:53:429:13615:-1220.76,-116.422,0 1/1:1300:4:69:1293:44256:-3977.02,-373.134,0
chrM 1438 . A G 79172.1 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=2474;CIGAR=1X;DP=2480;DPB=2480;DPRA=0;EPP=7.56039;EPPR=3.44459;GTI=0;LEN=1;MEANALT=1.5;MQM=59.8319;MQMR=58;NS=2;NUMALT=1;ODDS=1085.01;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=88621;QR=102;RO=5;RPL=1546;RPP=338.232;RPPR=3.44459;RPR=928;RUN=1;SAF=1055;SAP=119.304;SAR=1419;SRF=3;SRP=3.44459;SRR=2;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:551:1:16:550:19482:-1752.13,-161.526,0 1/1:1929:4:86:1924:69139:-6214.93,-560.827,0
chrM 2487 . A C 4432.57 . AB=0.278634;ABP=775.959;AC=1;AF=0.25;AN=4;AO=621;CIGAR=1X;DP=2340;DPB=2340;DPRA=0;EPP=15.1824;EPPR=99.2128;GTI=1;LEN=1;MEANALT=2.5;MQM=59.3285;MQMR=59.9115;NS=2;NUMALT=1;ODDS=63.7254;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=9402;QR=56614;RO=1707;RPL=281;RPP=15.1824;RPPR=127.637;RPR=340;RUN=1;SAF=0;SAP=1351.49;SAR=621;SRF=1352;SRP=1267.49;SRR=355;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/0:524:405:13274:115:1754:-119.206,0,-1156.18 0/1:1816:1302:43340:506:7648:-607.816,0,-3820.28
chrM 2706 . A G 49482.2 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=1889;CIGAR=1X;DP=1969;DPB=1969;DPRA=0;EPP=11.3157;EPPR=6.95112;GTI=0;LEN=1;MEANALT=2.5;MQM=59.9645;MQMR=59.5926;NS=2;NUMALT=1;ODDS=759.158;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=55923;QR=722;RO=27;RPL=810;RPP=86.1918;RPPR=5.02092;RPR=1079;RUN=1;SAF=802;SAP=96.3813;SAR=1087;SRF=18;SRP=9.52472;SRR=9;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:408:5:99:391:11529:-1028.82,-99.3894,0 1/1:1561:22:623:1498:44394:-3939.48,-352.569,0
chrM 3197 . T C 135699 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=4208;CIGAR=1X;DP=4241;DPB=4241;DPRA=0;EPP=168.325;EPPR=3.13803;GTI=0;LEN=1;MEANALT=3;MQM=59.9943;MQMR=60;NS=2;NUMALT=1;ODDS=2145.76;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=152221;QR=558;RO=17;RPL=2378;RPP=157.977;RPPR=6.20364;RPR=1830;RUN=1;SAF=1641;SAP=445.497;SAR=2567;SRF=8;SRP=3.13803;SRR=9;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:1499:6:179:1486:53236:-4775.26,-416.785,0 1/1:2742:11:379:2722:98985:-8874.65,-758.304,0
chrM 3243 . A G 46067 . AB=0.612338;ABP=290.859;AC=2;AF=0.5;AN=4;AO=1608;CIGAR=1X;DP=2626;DPB=2626;DPRA=0;EPP=31.0126;EPPR=64.3549;GTI=0;LEN=1;MEANALT=2;MQM=59.9627;MQMR=59.815;NS=2;NUMALT=1;ODDS=1288.98;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=53165;QR=35336;RO=1011;RPL=974;RPP=159.119;RPPR=763.402;RPR=634;RUN=1;SAF=558;SAP=329.898;SAR=1050;SRF=383;SRP=131.935;SRR=628;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/1:1068:221:7574:841:27395:-2380.4,0,-596.524 0/1:1558:790:27762:767:25770:-2317.69,0,-2496.98
chrM 3483 . G C 685.467 . AB=0.254386;ABP=182.214;AC=1;AF=0.25;AN=4;AO=127;CIGAR=1X;DP=550;DPB=550;DPRA=0;EPP=37.6342;EPPR=22.2028;GTI=1;LEN=1;MEANALT=1.5;MQM=59.4646;MQMR=59.8504;NS=2;NUMALT=1;ODDS=25.0865;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=2032;QR=13200;RO=421;RPL=87;RPP=40.7802;RPPR=245.89;RPR=40;RUN=1;SAF=1;SAP=270.17;SAR=126;SRF=321;SRP=254.927;SRR=100;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/0:208:166:5264:40:608:-35.5966,0,-454.8 0/1:342:255:7936:87:1424:-108.297,0,-694.524
chrM 3488 . T A 682.097 . AB=0.264706;ABP=166.509;AC=1;AF=0.25;AN=4;AO=130;CIGAR=1X;DP=546;DPB=546;DPRA=0;EPP=44.7694;EPPR=34.7681;GTI=1;LEN=1;MEANALT=1;MQM=59.4231;MQMR=59.7139;NS=2;NUMALT=1;ODDS=17.5994;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=2069;QR=13578;RO=416;RPL=90;RPP=44.7694;RPPR=211.806;RPR=40;RUN=1;SAF=0;SAP=285.302;SAR=130;SRF=315;SRP=242.06;SRR=101;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/0:206:166:5535:40:650:-39.5324,0,-479.353 0/1:340:250:8043:90:1419:-109.544,0,-705.868
chrM 3511 . A C 434.289 . AB=0.260394;ABP=230.901;AC=1;AF=0.25;AN=4;AO=185;CIGAR=1X;DP=752;DPB=752;DPRA=0;EPP=322.569;EPPR=29.1769;GTI=1;LEN=1;MEANALT=3;MQM=59.7838;MQMR=59.7348;NS=2;NUMALT=1;ODDS=57.1305;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=2698;QR=16673;RO=558;RPL=11;RPP=314.869;RPPR=115.475;RPR=174;RUN=1;SAF=1;SAP=396.094;SAR=184;SRF=292;SRP=5.64097;SRR=266;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/0:295:226:6735:66:923:-61.5611,0,-584.793 0/1:457:332:9938:119:1775:-135.644,0,-870.462
chrM 4769 . A G 54711.1 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=1746;CIGAR=1X;DP=1752;DPB=1752;DPRA=0;EPP=85.7949;EPPR=5.18177;GTI=0;LEN=1;MEANALT=2.5;MQM=51.2801;MQMR=58;NS=2;NUMALT=1;ODDS=911.774;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=61628;QR=15;RO=1;RPL=549;RPP=525.238;RPPR=5.18177;RPR=1197;RUN=1;SAF=1003;SAP=87.0833;SAR=743;SRF=1;SRP=5.18177;SRR=0;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:604:1:15:601:20766:-1867.77,-177.095,0 1/1:1148:0:0:1145:40862:-3677.79,-344.679,0
chrM 5539 . A G 11837 . AB=0.479167;ABP=6.26751;AC=2;AF=0.5;AN=4;AO=414;CIGAR=1X;DP=864;DPB=864;DPRA=0;EPP=192.358;EPPR=179.441;GTI=0;LEN=1;MEANALT=1.5;MQM=54.1957;MQMR=53.5924;NS=2;NUMALT=1;ODDS=622.768;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=14380;QR=15965;RO=449;RPL=85;RPP=315.283;RPPR=358.189;RPR=329;RUN=1;SAF=309;SAP=221.29;SAR=105;SRF=337;SRP=247.845;SRR=112;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/1:338:249:8721:89:3010:-252.807,0,-766.809 0/1:526:200:7244:325:11370:-1015.56,0,-644.23
chrM 7028 . C T 76141.7 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=2473;CIGAR=1X;DP=2499;DPB=2499;DPRA=0;EPP=3.74876;EPPR=34.9902;GTI=0;LEN=1;MEANALT=2.5;MQM=55.905;MQMR=57.6364;NS=2;NUMALT=1;ODDS=1210.59;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=85103;QR=439;RO=22;RPL=1260;RPP=4.94996;RPPR=4.58955;RPR=1213;RUN=1;SAF=1102;SAP=66.5485;SAR=1371;SRF=9;SRP=4.58955;SRR=13;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:827:6:107:820:28371:-2543.94,-224.334,0 1/1:1672:16:332:1653:56732:-5076.14,-434.286,0
chrM 7269 . G A 62196.6 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=1937;CIGAR=1X;DP=1947;DPB=1947;DPRA=0;EPP=54.8308;EPPR=5.18177;GTI=0;LEN=1;MEANALT=3;MQM=58.2685;MQMR=60;NS=2;NUMALT=1;ODDS=1033.49;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=69240;QR=16;RO=1;RPL=1011;RPP=11.1099;RPPR=5.18177;RPR=926;RUN=1;SAF=933;SAP=8.66151;SAR=1004;SRF=0;SRP=5.18177;SRR=1;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:704:1:16:698:24364:-2191.49,-206.139,0 1/1:1243:0:0:1239:44876:-4039.06,-372.976,0
chrM 8557 . G C 2590.97 . AB=0.267066;ABP=790.051;AC=2;AF=0.5;AN=4;AO=446;CIGAR=1X;DP=1670;DPB=1670;DPRA=0;EPP=44.2196;EPPR=97.7883;GTI=0;LEN=1;MEANALT=3;MQM=57.6951;MQMR=59.5256;NS=2;NUMALT=1;ODDS=125.064;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=6303;QR=38747;RO=1212;RPL=177;RPP=44.2196;RPPR=385.426;RPR=269;RUN=1;SAF=2;SAP=954.193;SAR=444;SRF=906;SRP=648.002;SRR=306;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/1:724:538:17225:181:2490:-182.373,0,-1508.7 0/1:946:674:21522:265:3813:-301.57,0,-1895.55
chrM 8860 . A G 55525 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=1846;CIGAR=1X;DP=1861;DPB=1861;DPRA=0;EPP=5.72052;EPPR=6.91895;GTI=0;LEN=1;MEANALT=3;MQM=47.1728;MQMR=58.6;NS=2;NUMALT=1;ODDS=1039.99;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=61929;QR=160;RO=5;RPL=984;RPP=20.5185;RPPR=6.91895;RPR=862;RUN=1;SAF=987;SAP=22.283;SAR=859;SRF=2;SRP=3.44459;SRR=3;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:729:0:0:726:24114:-2170.46,-218.548,0 1/1:1132:5:160:1120:37815:-3389.07,-311.012,0
chrM 9477 . G A 34109.5 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=1099;CIGAR=1X;DP=1104;DPB=1104;DPRA=0;EPP=9.42988;EPPR=3.0103;GTI=0;LEN=1;MEANALT=2;MQM=59.3794;MQMR=60;NS=2;NUMALT=1;ODDS=565.855;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=38032;QR=67;RO=2;RPL=598;RPP=21.6012;RPPR=7.35324;RPR=501;RUN=1;SAF=542;SAP=3.45487;SAR=557;SRF=1;SRP=3.0103;SRR=1;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:401:2:67:398:13308:-1191.76,-109.337,0 1/1:703:0:0:701:24724:-2225.39,-211.022,0
chrM 9548 . G A 26846.1 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=942;CIGAR=1X;DP=970;DPB=970;DPRA=0;EPP=3.04718;EPPR=3.73412;GTI=0;LEN=1;MEANALT=3;MQM=59.6921;MQMR=60;NS=2;NUMALT=1;ODDS=502.835;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=29956;QR=66;RO=3;RPL=524;RPP=28.9112;RPPR=3.73412;RPR=418;RUN=1;SAF=487;SAP=5.3708;SAR=455;SRF=3;SRP=9.52472;SRR=0;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:364:1:38:350:10822:-970.712,-99.6786,0 1/1:606:2:28:592:19134:-1719.73,-171.045,0
chrM 11467 . A G 164822 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=5200;CIGAR=1X;DP=5225;DPB=5225;DPRA=0;EPP=350.339;EPPR=4.78696;GTI=0;LEN=1;MEANALT=2.5;MQM=59.9342;MQMR=53.6364;NS=2;NUMALT=1;ODDS=2859.91;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=187277;QR=283;RO=11;RPL=3887;RPP=2769.75;RPPR=3.20771;RPR=1313;RUN=1;SAF=2257;SAP=199.527;SAR=2943;SRF=6;SRP=3.20771;SRR=5;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:2016:2:46:2008:71984:-6474.61,-594.606,0 1/1:3209:9:237:3192:115293:-10355.2,-916.222,0
chrM 11719 . G A 95624.7 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=3302;CIGAR=1X;DP=3356;DPB=3356;DPRA=0;EPP=179.466;EPPR=18.4661;GTI=0;LEN=1;MEANALT=2;MQM=59.5924;MQMR=58.2353;NS=2;NUMALT=1;ODDS=1506.69;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=106982;QR=483;RO=17;RPL=1766;RPP=37.7986;RPPR=6.20364;RPR=1536;RUN=1;SAF=1728;SAP=18.6065;SAR=1574;SRF=3;SRP=18.4661;SRR=14;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:911:4:122:891:28560:-2559.58,-247.982,0 1/1:2445:13:361:2411:78422:-7025.63,-662.959,0
chrM 12308 . A G 67204.7 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=2144;CIGAR=1X;DP=2161;DPB=2161;DPRA=0;EPP=8.55647;EPPR=3.87889;GTI=0;LEN=1;MEANALT=2;MQM=59.9664;MQMR=59.7;NS=2;NUMALT=1;ODDS=949.477;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=75192;QR=257;RO=10;RPL=1284;RPP=185.09;RPPR=3.87889;RPR=860;RUN=1;SAF=1005;SAP=21.1964;SAR=1139;SRF=4;SRP=3.87889;SRR=6;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:635:7:216:628:21603:-1924.87,-155.503,0 1/1:1526:3:41:1516:53589:-4819.52,-444.815,0
chrM 12372 . G A 62064 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=1984;CIGAR=1X;DP=1992;DPB=1992;DPRA=0;EPP=10.3697;EPPR=4.45795;GTI=0;LEN=1;MEANALT=2;MQM=59.9919;MQMR=60;NS=2;NUMALT=1;ODDS=933.299;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=69281;QR=192;RO=6;RPL=861;RPP=78.1406;RPPR=4.45795;RPR=1123;RUN=1;SAF=1010;SAP=4.42876;SAR=974;SRF=4;SRP=4.45795;SRR=2;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:634:5:155:628:21590:-1929.21,-164.556,0 1/1:1358:1:37:1356:47691:-4288.95,-401.925,0
chrM 13617 . T C 28593.6 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=901;CIGAR=1X;DP=906;DPB=906;DPRA=0;EPP=251.346;EPPR=3.73412;GTI=0;LEN=1;MEANALT=2;MQM=59.9034;MQMR=60;NS=2;NUMALT=1;ODDS=462.343;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=32868;QR=92;RO=3;RPL=674;RPP=484.564;RPPR=9.52472;RPR=227;RUN=1;SAF=339;SAP=122.861;SAR=562;SRF=2;SRP=3.73412;SRR=1;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:303:1:14:301:10938:-983.358,-87.1961,0 1/1:603:2:78:600:21930:-1966.72,-168.809,0
chrM 14766 . C T 60668.6 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=2022;CIGAR=1X;DP=2039;DPB=2039;DPRA=0;EPP=13.3243;EPPR=19.0002;GTI=0;LEN=1;MEANALT=2.5;MQM=59.9782;MQMR=60;NS=2;NUMALT=1;ODDS=954.02;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=67719;QR=140;RO=11;RPL=989;RPP=5.08941;RPPR=12.6832;RPR=1033;RUN=1;SAF=1199;SAP=154.837;SAR=823;SRF=1;SRP=19.0002;SRR=10;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:637:6:78:628:20191:-1810.35,-169.906,0 1/1:1402:5:62:1394:47528:-4272.15,-401.924,0
chrM 14793 . A G 58080 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=1967;CIGAR=1X;DP=1998;DPB=1998;DPRA=0;EPP=8.57532;EPPR=5.80219;GTI=0;LEN=1;MEANALT=3;MQM=59.9736;MQMR=59.2857;NS=2;NUMALT=1;ODDS=930.516;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=64876;QR=104;RO=7;RPL=1133;RPP=101.705;RPPR=3.32051;RPR=834;RUN=1;SAF=1124;SAP=90.1794;SAR=843;SRF=1;SRP=10.7656;SRR=6;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:600:4:62:589:19341:-1735.29,-163.219,0 1/1:1398:3:42:1378:45535:-4094.54,-403.304,0
chrM 15301 . G A 76440.4 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=2590;CIGAR=1X;DP=2644;DPB=2644;DPRA=0;EPP=3.76487;EPPR=7.94546;GTI=0;LEN=1;MEANALT=3;MQM=60;MQMR=60;NS=2;NUMALT=1;ODDS=1170.15;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=85385;QR=292;RO=11;RPL=1134;RPP=89.9396;RPPR=4.78696;RPR=1456;RUN=1;SAF=1194;SAP=37.2206;SAR=1396;SRF=3;SRP=7.94546;SRR=8;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:726:5:116:709:23434:-2098.78,-192.286,0 1/1:1918:6:176:1881:61951:-5559.91,-535.386,0
chrM 15326 . A G 79542.1 . AB=0;ABP=0;AC=4;AF=1;AN=4;AO=2574;CIGAR=1X;DP=2586;DPB=2586;DPRA=0;EPP=3.76956;EPPR=5.18177;GTI=0;LEN=1;MEANALT=3;MQM=60;MQMR=60;NS=2;NUMALT=1;ODDS=1207.35;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=88636;QR=86;RO=4;RPL=1116;RPP=101.683;RPPR=5.18177;RPR=1458;RUN=1;SAF=1198;SAP=29.7395;SAR=1376;SRF=0;SRP=11.6962;SRR=4;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 1/1:715:2:50:710:24322:-2184.63,-204.389,0 1/1:1871:2:36:1864:64314:-5785.22,-552.228,0
Filtering VCF data
Even though we selected somewhat stringent input parameters (restricting base quality to a minimum of 30 and mapping quality to a minimum of 20) there is still a lot of just in our data. Erik Garrison has a beautiful illustration of various biases potentially affecting called variants (and making a locus sequence-able):
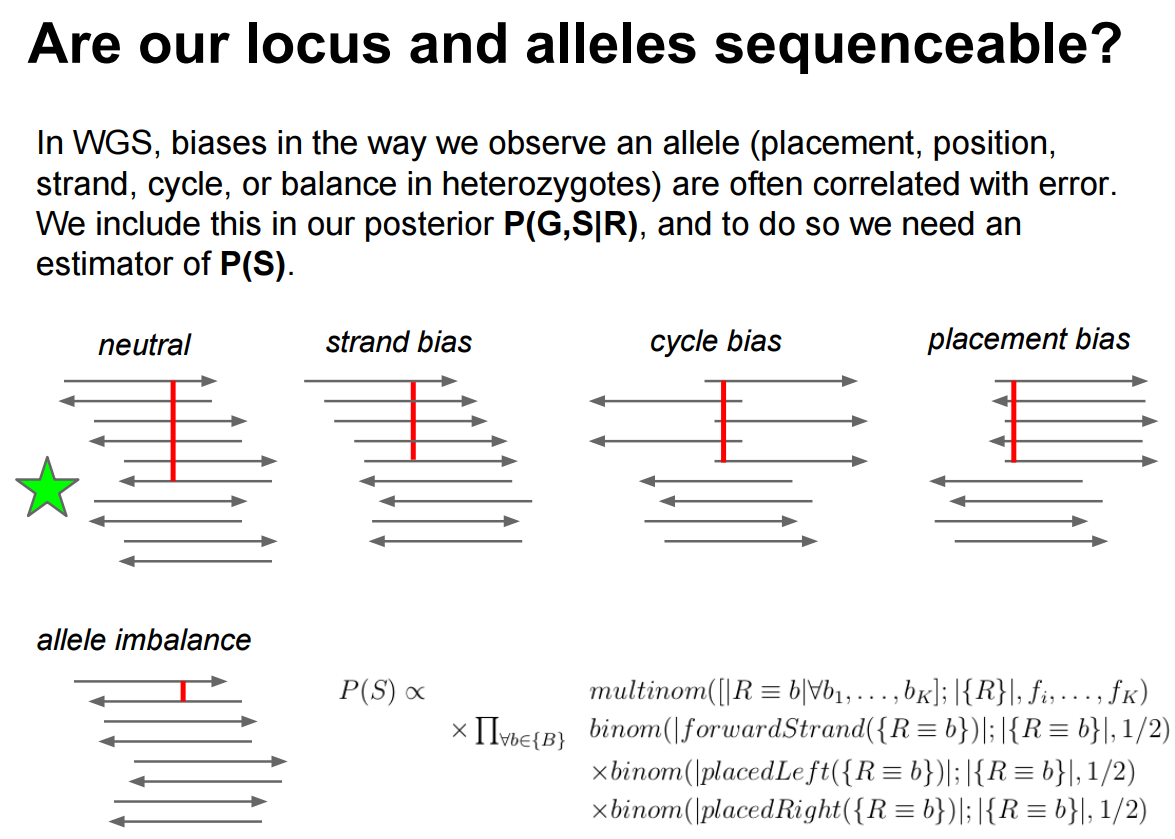 |
| Here you can see that in an ideal case (indicated with a green star) a variant is evenly represent by different areas of sequencing reads (cycle and placement biases) and is balanced across the two strands (strand bias). Allele imbalance is not applicable in our case as it reflects significant deviation from the diploid (50/50) expectation (see here for more details). |
A robust tool set for processing VCF data is provided by vcflib developed by Erik Garrison, the author of FreeBayes. One way to filter VCF is using INFO fields of the VCF dataset. If you look at the VCF dataset shown above you will see all comment lines beginning with ##INFO. These are INFO fields. Each VCF record contains a list of INFO tags describing a wide range of properties for each VCF record. You will see that FreeBayes and NVC differ significantly in the number and types of INFO fields each of these caller generates. This why the two require different filtering strategies.
Among numerous types of data generated by FreeBayes let's consider the following variant properties:
##INFO=<ID=DP,Number=1,Type=Integer,Description="Total read depth at the locus">This is simply the number of reads covering a given site.##INFO=<ID=SRP,Number=1,Type=Float,Description="Strand balance probability for the reference allele: Phred-scaled upper-bounds estimate of the probability of observing the deviation between SRF and SRR given E(SRF/SRR) ~ 0.5, derived using Hoeffding's inequality">The higher this quantity the better the site as it diminishes the chances of the sites having significant strand bias.##INFO=<ID=SAP,Number=A,Type=Float,Description="Strand balance probability for the alternate allele: Phred-scaled upper-bounds estimate of the probability of observing the deviation between SAF and SAR given E(SAF/SAR) ~ 0.5, derived using Hoeffding's inequality">The higher this quantity the better the site as it diminishes the chances of the sites having significant strand bias (also see here).##INFO=<ID=EPP,Number=A,Type=Float,Description="End Placement Probability: Phred-scaled upper-bounds estimate of the probability of observing the deviation between EL and ER given E(EL/ER) ~ 0.5, derived using Hoeffding's inequality">The higher this number the lower the chance of having significant placement bias.QUAL- phred scaled variant quality.
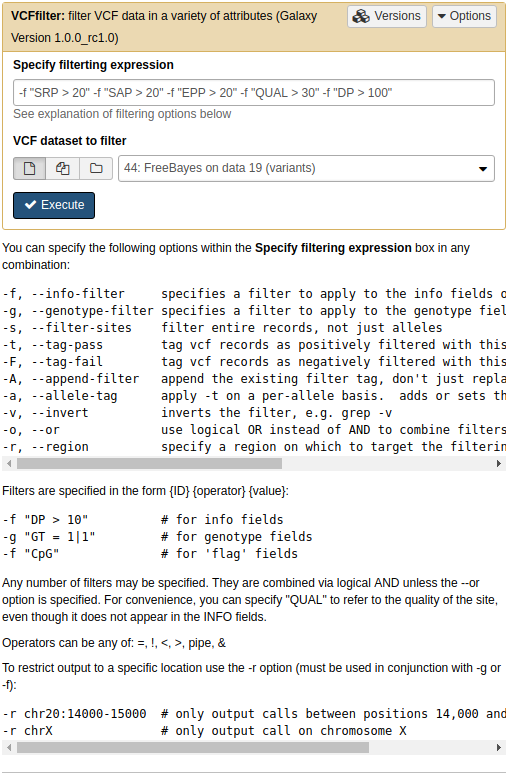
To perform filtering we will use NGS: VCF Manipulation → VCFfilter):
Filtering VCF data
Filtering FreeBayes VCF for strand bias (
SPRandSAP), placement bias (EPP), variant quality (QUAL), and depth of coverage (DP).
The resulting VCF only contains five variants (most comments fields are omitted here):
#CHROM POS ID REF ALT QUAL FILTER INFO FORMAT raw_child-ds- raw_mother-ds-
chrM 3243 . A G 46067 . AB=0.612338;ABP=290.859;AC=2;AF=0.5;AN=4;AO=1608;CIGAR=1X;DP=2626;DPB=2626;DPRA=0;EPP=31.0126;EPPR=64.3549;GTI=0;LEN=1;MEANALT=2;MQM=59.9627;MQMR=59.815;NS=2;NUMALT=1;ODDS=1288.98;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=53165;QR=35336;RO=1011;RPL=974;RPP=159.119;RPPR=763.402;RPR=634;RUN=1;SAF=558;SAP=329.898;SAR=1050;SRF=383;SRP=131.935;SRR=628;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/1:1068:221:7574:841:27395:-2380.4,0,-596.524 0/1:1558:790:27762:767:25770:-2317.69,0,-2496.98
chrM 3483 . G C 685.467 . AB=0.254386;ABP=182.214;AC=1;AF=0.25;AN=4;AO=127;CIGAR=1X;DP=550;DPB=550;DPRA=0;EPP=37.6342;EPPR=22.2028;GTI=1;LEN=1;MEANALT=1.5;MQM=59.4646;MQMR=59.8504;NS=2;NUMALT=1;ODDS=25.0865;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=2032;QR=13200;RO=421;RPL=87;RPP=40.7802;RPPR=245.89;RPR=40;RUN=1;SAF=1;SAP=270.17;SAR=126;SRF=321;SRP=254.927;SRR=100;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/0:208:166:5264:40:608:-35.5966,0,-454.8 0/1:342:255:7936:87:1424:-108.297,0,-694.524
chrM 3488 . T A 682.097 . AB=0.264706;ABP=166.509;AC=1;AF=0.25;AN=4;AO=130;CIGAR=1X;DP=546;DPB=546;DPRA=0;EPP=44.7694;EPPR=34.7681;GTI=1;LEN=1;MEANALT=1;MQM=59.4231;MQMR=59.7139;NS=2;NUMALT=1;ODDS=17.5994;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=2069;QR=13578;RO=416;RPL=90;RPP=44.7694;RPPR=211.806;RPR=40;RUN=1;SAF=0;SAP=285.302;SAR=130;SRF=315;SRP=242.06;SRR=101;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/0:206:166:5535:40:650:-39.5324,0,-479.353 0/1:340:250:8043:90:1419:-109.544,0,-705.868
chrM 5539 . A G 11837 . AB=0.479167;ABP=6.26751;AC=2;AF=0.5;AN=4;AO=414;CIGAR=1X;DP=864;DPB=864;DPRA=0;EPP=192.358;EPPR=179.441;GTI=0;LEN=1;MEANALT=1.5;MQM=54.1957;MQMR=53.5924;NS=2;NUMALT=1;ODDS=622.768;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=14380;QR=15965;RO=449;RPL=85;RPP=315.283;RPPR=358.189;RPR=329;RUN=1;SAF=309;SAP=221.29;SAR=105;SRF=337;SRP=247.845;SRR=112;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/1:338:249:8721:89:3010:-252.807,0,-766.809 0/1:526:200:7244:325:11370:-1015.56,0,-644.23
chrM 8557 . G C 2590.97 . AB=0.267066;ABP=790.051;AC=2;AF=0.5;AN=4;AO=446;CIGAR=1X;DP=1670;DPB=1670;DPRA=0;EPP=44.2196;EPPR=97.7883;GTI=0;LEN=1;MEANALT=3;MQM=57.6951;MQMR=59.5256;NS=2;NUMALT=1;ODDS=125.064;PAIRED=1;PAIREDR=1;PAO=0;PQA=0;PQR=0;PRO=0;QA=6303;QR=38747;RO=1212;RPL=177;RPP=44.2196;RPPR=385.426;RPR=269;RUN=1;SAF=2;SAP=954.193;SAR=444;SRF=906;SRP=648.002;SRR=306;TYPE=snp;technology.ILLUMINA=1 GT:DP:RO:QR:AO:QA:GL 0/1:724:538:17225:181:2490:-182.373,0,-1508.7 0/1:946:674:21522:265:3813:-301.57,0,-1895.55
Looking at the data
For visalizaning VCFs Galaxy relies on the two external tools. The first is called VCF.IOBIO and is developed by Gabor Marth's group at the University of Utah. The second is called IGV developed by Broad Institute.
VCF.IOBIO
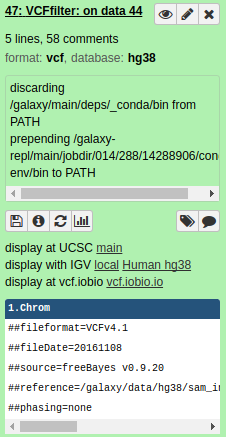
VCF.IOBIO can be invoked by expanding a VCF dataset in Galaxy's history by clicking on it:
Displaying data in VCF.IOBIO
Clicking on the dataset above will expand it as shown below:
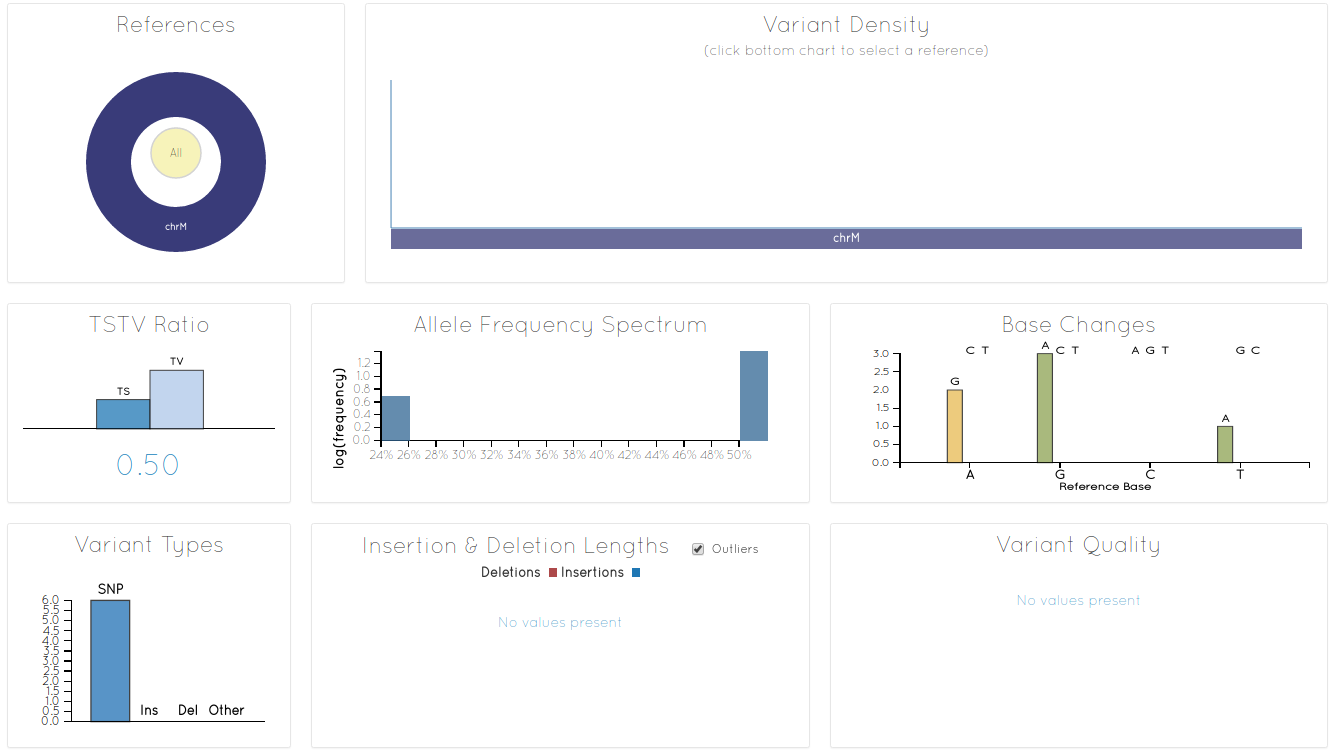
At the bottom there is a link "display at vcf.iobio" Clicking on this link will start indexing of VCF datasets, which is required to display them. After indexing VCF.IOBIO will open:
Of course there are not that many variants to look at in this example. Nevertheless there are helpful statistics such as Transition/Transversion (Ts/Tn) ratio.
IGV
Similarly to VCF.BIOIO expanding a history item representing a VCF dataset will reveal an IGV link:
Displaying data in IGV
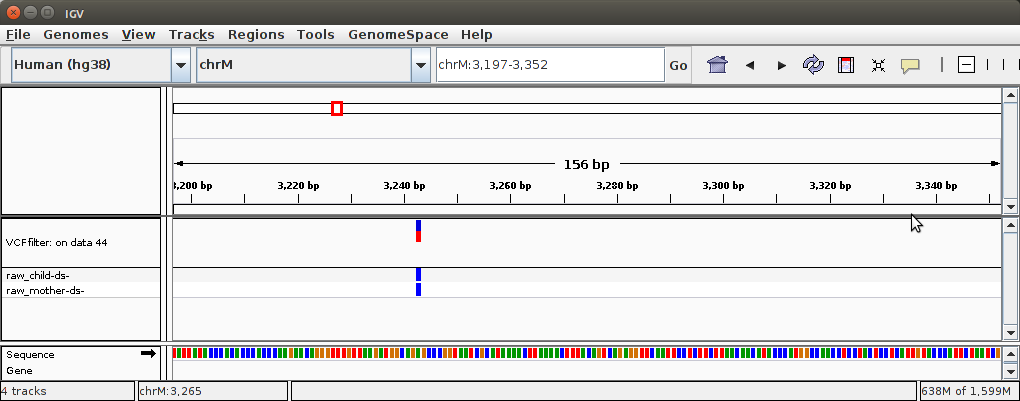
At the bottom there is a link "display at IGV: local Human hg38" The difference between "local" and "Human hg38" links is explained in the following video:
Visualizing our FreeBayes dataset will produce this:
Here we focus on one particular variant at position 3,243 for reasons that will become apparent in the next section.
Digging into the data
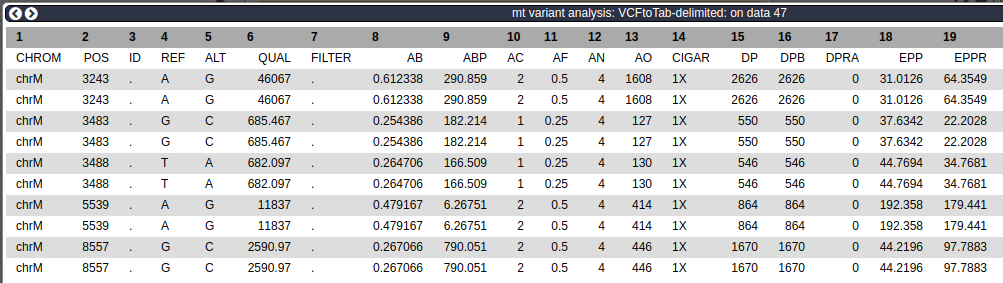
Visualizing VCF dataset may be a good way to get an overall idea of the data, but it does not tell a lot of details. For example, above we have visualized site 3,243 using IGV. It is interesting but we need to find out more. One thing we can do is to convert VCF dataset into a tab-delimited representation and play a bit more with it.
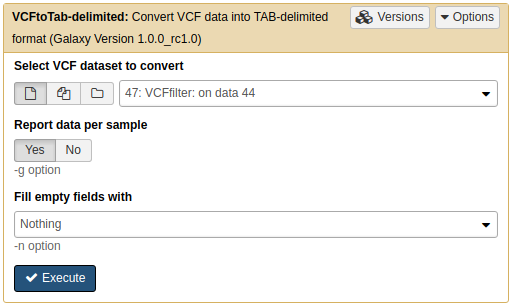
Using NGS: VCF Manipulation → VCFtoTab-delimited on the filtered VCF dataset:
From VCF to Tab-delimited data
Make sure Report data per sample is set to
YesThis will produce a dataset with very many columns:
There are 53 columns in this dataset (not all are shown here).
The columns in the above dataset represent INFO and Genotype fields on the original VCF dataset. Let's restrict ourselves to just a few:
- 2
POS- position along mitochondrial genome - 4
REF- reference allele - 5
ALT- alternative allele - 50
SAMPLE- name of the sample - 51
AO- number of alternative observations (how many times do we see the alternative allele at this position in this sample) - 52
DP- depth of coverage at this site for this sample
To cut these columns out we will use Text Manipulation → Cut
Cutting columns from a file
Note that column names are pre-ceded with
cThis will generate the following dataset:
POS REF ALT SAMPLE AO DP -------------------------------------- 3243 A G raw_child-ds- 841 1068 3243 A G raw_mother-ds- 767 1558 3483 G C raw_child-ds- 40 208 3483 G C raw_mother-ds- 87 342 3488 T A raw_child-ds- 40 206 3488 T A raw_mother-ds- 90 340 5539 A G raw_child-ds- 89 338 5539 A G raw_mother-ds- 325 526 8557 G C raw_child-ds- 181 724 8557 G C raw_mother-ds- 265 946
Let's look at site 4,243. At this site Mother has 841 Gs (since G is an alternative allele) and 1,068-841=227 As. This child has 767 Gs and 1,558-767=791 As:
Allele A G
-------------------
Mother 227 841
Child 791 767
Thus the major allele in mother (G) becomes the minor allele in child -- a remarkable frequency change due to mitochondrial bottleneck!
Take a look at the whole thing
This entire analysis is available as a Galaxy history that you can import into your Galaxy account and play with.
Now you know how to call variants in non-diploid system, so try it on bacteria, viruses etc...
Exercise
Time to really do it yourself. Please, complete the following exercise:
Find variants in a virus
Suppose you obtained a virus from some source and you would like to see how it is different from its published reference sequence. You have sequenced the virus and obtained two Illumina files (these files are large, so don't open them. Rather copy their addresses (right click) and use them to upload into Galaxy as explained in Hints section below):
Analyze these files using Galaxy as was explained in this lesson by mapping them against this reference genome (again right click to copy the address); see Tips).
Tips:
- You need to upload reads and the reference genome into Galaxy (http://usegalaxy.org) as shown in this video
- You will be mapping reads against an uploaded reference genome as shown in this video
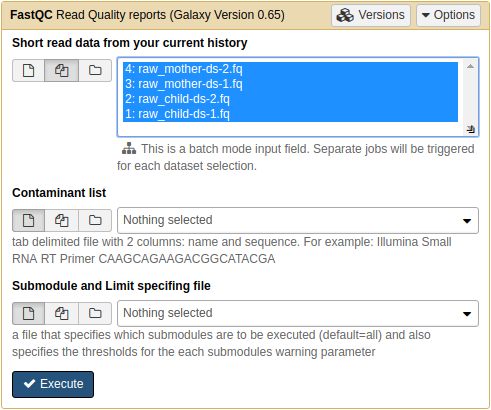
 adjacent to the Short read data from your current history widget. Once
adjacent to the Short read data from your current history widget. Once